Li-ion Batteries
Significant part of our research is currently funded by the DOE BATT program and Collaborative Reserach Alliance with Army Research Laboratory to conduct multiscale modeling of materials for application in Li-ion batteries.
In the current generation of lithium ion batteries, the solid electrolyte interface (SEI) at the anode/electrolyte interface formed from reduction of the electrolyte and the passivation layer at the cathode/electrolyte interface formed by oxidation of the electrolyte and anion are critical components in determingthe performance and lifetime of the cell. For example, the SEI layer stabilizes the electrode against solvent intercalation/dissolution and stabilizes the electrolyte against electrochemical decomposition. Formation of SEI layers with good transport properties, good mechanical properties and electrochemical stability is of paramount importance in obtaining good cell performance and cycling. While it is known that interfacial resistance to charge transport dominates overall cell resistance at lower temperature, but it is unclear as to why. Furthermore, it is known that additives can improve SEI performance, but the mechanism, and in general mechanisms of side reactions at the cathode and anode surfaces, are not understood. And while evidence is mounting that nanoscale morphology is important in determining the behavior of the cathode and anode and that control of morphology may be key to improved battery performance, the lack of understanding of the connection between morphology and performance precludes a systematic approach to optimizing battery performance. Hence, it is clear that the state-of-the-art in understanding the chemistry and physics of secondary lithium batteries is incomplete and largely empirical.
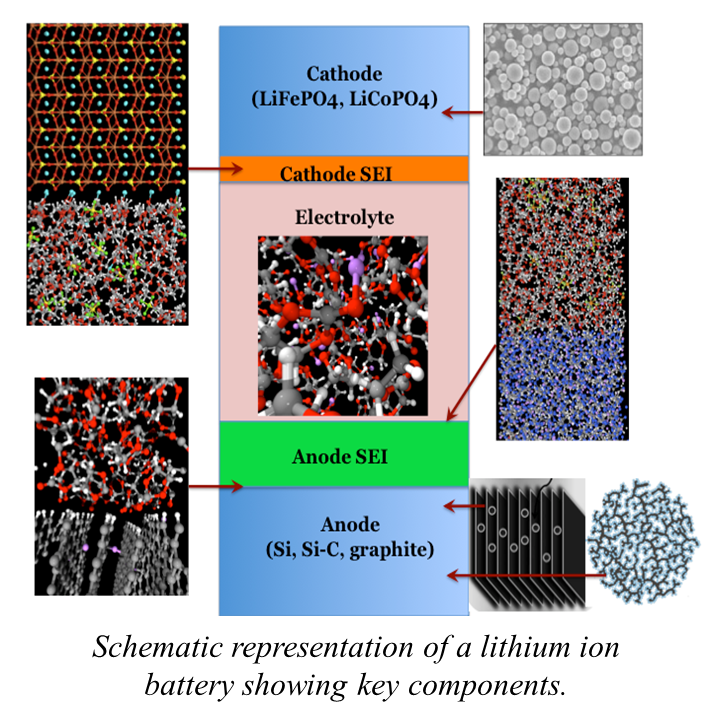
In these projects we employ multiscale modeling approach that requires integration of methods on scales ranging from the electronic structure level to the continuum level. Specifically we utilize:
1) ab initio electronic structure methods to obtain fundamental information about electrolyte oxidation and reduction reactions and well as the molecular-level structure and interactions within the electrolyte and polymer binder.
2) molecular dynamics simulations utilizing reactive force fields (ReaxFF) are used to investigate the condensed-phase oxidation and reduction reactions at the interface of the electrolyte with the cathode and anode, allowing us to elucidate mechanisms of formation and predict structure of the SEI.
3) molecular dynamics simulations utilizing highly-accurate polarizable, non-reactive force fields are used to predict the lithium transport properties of bulk electrolyte, SEI layers and cathode binder, as well as the interfacial (SEI/electrolyte and SEI/electrode) transport properties.
4) continuum level Material Point Method simulations are utilized in conjunction with reaction/diffusion models to predict the performance of the composite cathode as well as the mechanical properties of the anode SEI.